A quick note: this week’s post is a bit of a science challenge and requires some further investigation by the reader. Please click through to the links and email me if you have any questions.
The Energy Storage Association (ESA) lists the following electric storage technologies:
- Solid State Batteries – a range of electrochemical storage solutions, including advanced chemistry batteries and capacitors (Electrochemical Capacitors, Lithium Ion (Li-Ion) Batteries, Nickel-Cadmium (Ni-Cd) and Sodium Sulfur (NAS) Batteries)
- Flow Batteries – batteries where the energy is stored directly in the electrolyte solution for longer cycle life, and quick response times (redox Flow Batteries, Iron-Chromium (ICB Flow Batteries, Vanadium Redox (VRB) Flow Batteries and Zinc-Bromine (ZNBR) Flow Batteries)
- Flywheels – mechanical devices that harness rotational energy to deliver instantaneous electricity
- Compressed Air Energy Storage – utilizing compressed air to create a potent energy Reserve (Compress Air Energy Storage (AA-CAES), Advanced Adiabatic Compressed Air Energy Storage (AA-CAES) and Isothermal CAES)
- Thermal – capturing heat and cold to create energy on demand (Pumped Heat Electrical Storage (PHES), Hydrogen Energy Storage and Liquid Air Energy Storage (LAES) )
- Pumped Hydro-Power – creating large-scale reservoirs of energy with water (Pumped Hydroelectric Storage, Sub-Surface Pumped Hydroelectric Storage, Surface Reservoir Pumped Hydroelectric Storage and Variable Speed Pumped Hydroelectric Storage)
The technical ESA’s website also presents details of each storage method. There are, however – to use a common college term – some “prerequisites” necessary before we can follow up on these details. That kind of targeted educational background is something that most of us do not have. Since describing it in terms that don’t require such prerequisites requires a lot of effort and takes great deal of time for both the reader and writer, I will skip most of the explanations here. Anyone who wants to learn more about these storage modes is welcome to try his/her hand on the appropriate Wikipedia sites (If you hadn’t noticed yet, I tend to like Wikipedia as a source for quick, well-written information). Instead, for the moment, I will focus on two important categories: Solid State Batteries and Pumped Hydro-Power.
Solid State Batteries:
On a hot summer weekend, my wife and I were invited to visit friends in suburban NYC. The friends live in a house with a swimming pool and they invited us to take advantage of it. The gathering was pleasant and intimate, with us the only guests. I was sort of swimming around alone in the pool with everybody else chatting outside. Suddenly, the hostess called to me, saying that they needed some technical advice. The discussion topic was the cost of upkeep of the pool. They all agreed that it is ridiculously expensive and they were trying to find a better solution. My hostess got a proposal to save on chlorination by using a common salt and she asked me how it works. It had been a long time since I had to deal with swimming pool maintenance so I had to explain, using basic chemistry, that the only way that I can think of that it would work is through the use of electricity in an electrolytic process. Therefore, in order to figure out if the new system is a money saver, we will have to figure out if what she saves on chlorination will not be balanced out by the combination of an extra-large electric bill and the prices of the electrolyzer and regulator (more than $2,000). She shook her head and said the only thing that she knows about such matters (she is a college educated teacher) are the + and – in her car battery. Here is what Wikipedia says about the chlorination gadget:
Salt water chlorination is a process that uses dissolved salt (2,500–6,000 ppm) as a store for the chlorination system. The chlorine generator (also known as salt cell, salt generator, salt chlorinator) uses electrolysis in the presence of dissolved salt (NaCl) to produce hypochlorous acid (HCIO) and sodium hypochlorite (NaClO), which are the sanitizing agents already commonly used in swimming pools. As such, a saltwater pool is not actually chlorine-free; it simply utilizes a chlorine generator instead of direct addition of chlorine
Since I’m using her as a representative of general public knowledge, I guess we had better start with what she knows. Probably, the simplest battery that all of us know about is the Lead-Acid battery that are in use in most cars. I also found that this battery probably offers the simplest explanation how batteries in general work. The Lead-Acid is not even listed in the ESA list of batteries. If we look for a battery entry in Google and read the Wikipedia entry we find 20 different batteries (not the mere 4 shown on the ESA list). The working principle is similar to that of the Lead-Acid battery but the chemistry is different. Here is the simplest example that I could find of how the Lead-Acid battery works:
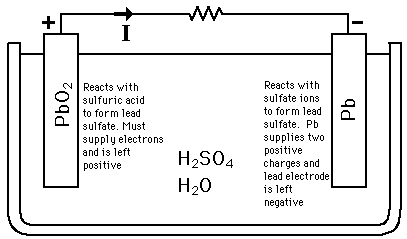 For reference, the compounds above are as follows: H2SO4 – Sulfuric Acid, PbO2 – Lead Oxide, PbSO4 – Lead Sulfate, H2O –Water, Pb – Lead.
For reference, the compounds above are as follows: H2SO4 – Sulfuric Acid, PbO2 – Lead Oxide, PbSO4 – Lead Sulfate, H2O –Water, Pb – Lead.
In the charging process Lead (Pb) is deposited from the Lead Sulfate (PbSO4) onto the negative electrode and at the same time Lead Oxide (PbO2) is deposited on the positive electrode. In the discharge process the reverse reactions take place. We use excess electricity to charge the battery and use this electricity when we have access demand. For a more detailed walk-through of the chemical process, you can go here.
Hydro-Power Storage:
Here the science is considerably simpler. A schematic is shown in the picture below: 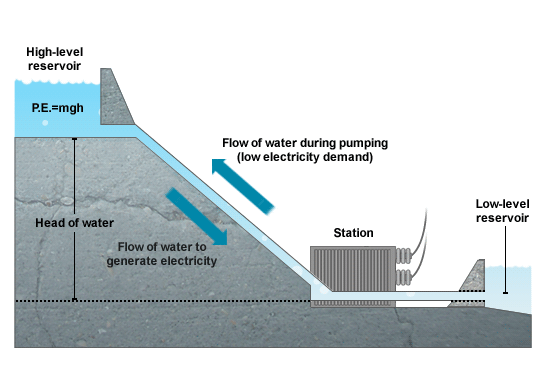 For storage we use electrical power to pump water to a reservoir uphill and when we need the extra power we use the stored water to run the power station below like a regular hydroelectric generation.
For storage we use electrical power to pump water to a reservoir uphill and when we need the extra power we use the stored water to run the power station below like a regular hydroelectric generation.
How do we convert the water flow into electricity? The process is similar to that of generating electricity from power plants run on fossil fuels such as coal and natural gas. In both cases we turn a propeller like a turbine that moves a magnet relative to conducting wires. This phenomenon produces electric power in a way that was originally demonstrated by the English scientist Michael Faraday (1791- 1867). Water is the most widely used storage device for power generating facilities but other materials can be used for the same purpose. Perhaps one of the most interesting variants is based on a loaded train that is driven uphill powered by access electricity and downhill to generate electricity when needed. The stored energy can be adjusted by changing the weight loaded. It can operate in places where hydroelectric storage is not practical.
The discussion with my friend about the cost-effectiveness of salt water chlorination is ongoing, but I will let you know when we reach a conclusion.
In future blogs I will focus on some of the economic considerations when choosing what storage devices to use and how to incorporate them into the grid structure.
* I always welcome questions and comments about my blog, my book, and my work in general. Unfortunately, I have been deluged with spam – both in the comments section here, and in my email. In the interest of spending my time addressing actual messages (instead of sorting through junk), I ask that you please send any questions to one of the following addresses, with the title, “Comment about CCF blog.”
micha (no space) tom (at) brooklyn (dot) cuny (dot) edu
or info (at) lcgcommunications (dot) com. Thank you for your continued readership and your feedback.
